TODAYS NOTES
STEPS:
1. Identify the type ofreaction (Rxn)
2. Put the products together
3. Put in subscripts inproducts
4. Balance the equation
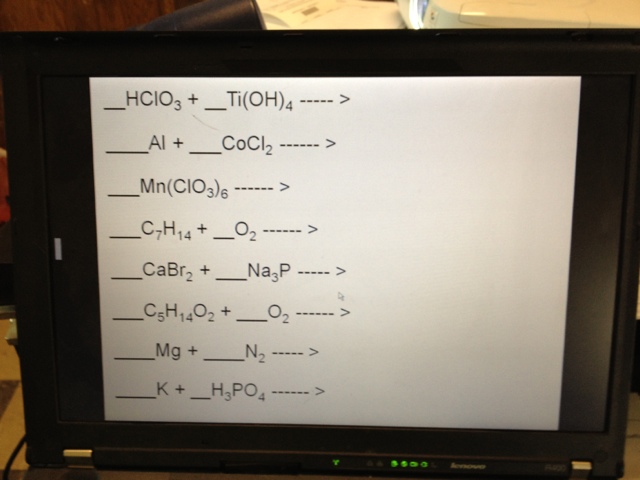
Completeand balance the equations
Problem: Ca + F2 ---à
Step1: Synthesis Type Reaction
Step2: Ca + F2 ---à CaF
Step3: Ca + F2 ---à CaF2
Step4: 1 Ca + F2 ---à 1 CaF2
Problem: V(OH)5 ---à
Step1: Decomposition
Step2: V(OH)5 ---àVO + 5H2O
Step3: V(OH)5 ---àV2O5 + 5H2O
Step4: 2 V(OH)5 ---àV2O5 + 5H2O
Problem: H3PO4+ Mg(OH)2 --à
Step1: Double Replacement: Acid Base
Step2: H3PO4 + Mg(OH)2 --à Mg (PO4) + HOH
Step3: H3PO4 + Mg(OH)2 --à Mg3 (PO4)2 + HOH
Step4: 2 H3PO4 + Mg(OH)2 --à Mg3 (PO4)2 + 6 HOH
Problem: Bi2(SO4)5 + Ga --à
Step1: Single Replacement Reaction
Step2: Bi2(SO4)5 + Ga --à Ga(SO4) + Bi
Step3: Bi2(SO4)5 + Ga --à Ga2(SO4)3 + Bi
Step4: 3Bi2(SO4)5 + 10Ga --à 5Ga2(SO4)3 + 6Bi
Problem: C4H7O2 +O2 --à
Step1: Combustion (carbohydrate)
Step2 C4H7O2 + O2 --à CO2 + H2O
Step3: C4H7O2 + O2 --à CO2 + H2O
Step4 4C4H7O2 + 19O2 --à 16CO2 + 14H2O
Problem: Ca(NO3)2 + Zn --à
Step1: Single Replacement Reaction
Step2: Because Zn is below Ca on the Activity Series
Sumof the coefficients: ZERO because they will not react.
Problem: Na2CO3(aq) + ZnF2(aq) --à
Step1: Double Replacement Reactions
Step2: Na2CO3(aq) + ZnF2 (aq) --à ZnCO3 + NaF
Step3: Na2CO3(aq) + ZnF2 (aq) --à ZnCO3 + NaF
Step4: Na2CO3(aq) + ZnF2 (aq) --à ZnCO3 + 2 NaF



No comments:
Post a Comment